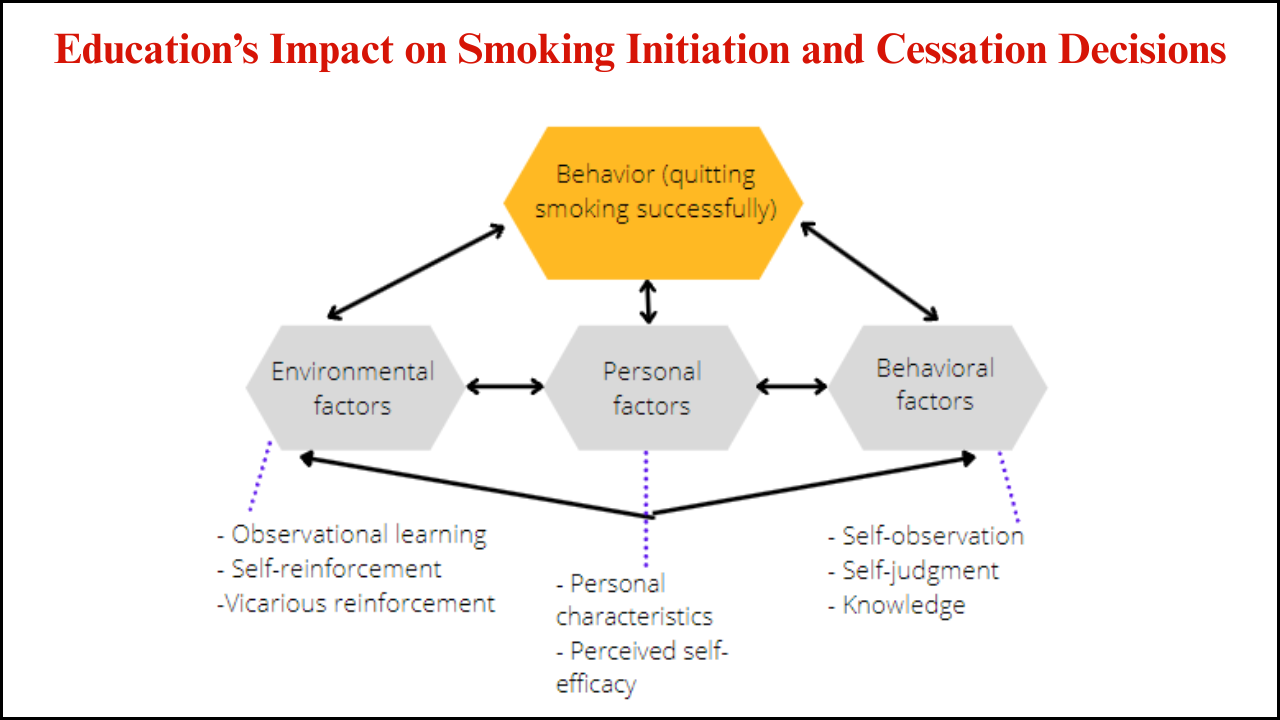
The Family Smoking Prevention and Tobacco Control Act of 2009 marked a turning point in American public health policies. This legislation gave the U.S. Food and Drug Administration (FDA) the authority to regulate the manufacturing, distribution, and marketing of tobacco products. The law aimed to reduce youth smoking, improve consumer awareness, and place stricter controls on an industry long criticized for manipulative practices. Understanding the impacts of this act requires examining its effects on public health, industry practices, regulatory mechanisms, and society at large.
Table of Contents
Key Objectives of the Act
- Empower the FDA to oversee tobacco regulation
- Restrict youth access to tobacco products
- Curb misleading advertising and labeling by tobacco companies
- Mandate disclosure of harmful ingredients
- Introduce warning labels on packaging and advertisements
- Promote scientific research on tobacco-related health risks
Public Health Impacts
- Smoking initiation among youth declined after stricter marketing and sales restrictions.
- Warning labels improved awareness of tobacco’s health risks among consumers.
- Bans on flavored cigarettes reduced appeal to young users.
- FDA’s power to evaluate new products helped prevent misleading claims such as “light” or “mild” cigarettes being healthier.
- Nicotine addiction prevention became a central goal of national health strategies.
Impacts on Tobacco Industry
- Manufacturers faced tighter restrictions on marketing strategies.
- Companies were prohibited from using terms like “low tar” or “light.”
- A greater financial burden was created due to compliance costs with FDA regulations.
- Market innovations, such as flavored products, faced regulatory hurdles.
- Industry influence on youth-oriented advertising significantly decreased.
Consumer Awareness and Behavior Changes
- Health warnings increased consumer knowledge about risks.
- Packaging restrictions reduced brand appeal.
- Smokers faced stronger incentives to quit due to visible risks.
- Public education campaigns reinforced FDA measures.
- Behavioral studies indicated a gradual decline in smoking prevalence post-legislation.
Major Provisions of the Act and Their Impacts
| Provision | Details | Impact on Public | Impact on Industry |
|---|---|---|---|
| FDA Authority | The FDA gained the power to regulate tobacco products | Increased trust in public health protections | Compliance costs and reduced autonomy |
| Flavor Ban | Ban on flavored cigarettes (except menthol initially) | Reduced youth appeal | Loss of flavored product markets |
| Warning Labels | Large, graphic warnings required on packs and ads | Higher awareness of health risks | Restricted branding and design freedom |
| Advertising Restrictions | Ban on misleading claims like “light” or “mild” | Clearer consumer understanding | Marketing strategies severely limited |
| Ingredient Disclosure | Companies must disclose product ingredients | Transparency for consumers | Increased regulatory oversight |
| New Product Review | FDA approval is required before new products enter the market | Protection from harmful innovations | Delay in product launches |
Youth Protection Measures
- Age verification requirements reduced illegal sales to minors.
- Point-of-sale restrictions limited youth exposure to advertisements.
- Removal of candy and fruit-flavored cigarettes curtailed early smoking initiation.
- School and community programs aligned with federal policy to discourage tobacco use.
- Tobacco companies could no longer sponsor youth-oriented cultural or sporting events.
Youth-Focused Outcomes
| Youth Measure | Purpose | Observed Impact |
|---|---|---|
| Age verification rules | Prevent sales to minors | Decrease in underage tobacco access |
| Flavor bans | Reduce appeal to youth | Lower initiation rates |
| Sponsorship restrictions | Block tobacco visibility in events | Reduced normalization of smoking |
| Ad restrictions near schools | Minimize exposure | Fewer children are exposed to marketing |
| Public education campaigns | Encourage prevention | Stronger awareness of risks |
Regulatory and Legal Outcomes
- FDA developed a structured process for evaluating new products.
- Litigation between tobacco companies and FDA increased, often challenging restrictions.
- States gained stronger support for local anti-smoking initiatives.
- Federal oversight standardized rules across the nation.
- Industry lobbying remained powerful, slowing some regulatory expansions.
Economic Impacts
- Tobacco sales declined gradually, reducing industry profits.
- Healthcare costs associated with smoking-related diseases showed signs of long-term reduction.
- Tax revenues from tobacco continued but shifted toward public health funding.
- Compliance costs increased for manufacturers, especially smaller firms.
- Job shifts occurred within the tobacco industry due to regulatory changes.
Scientific and Research Outcomes
- Funding increased for studies on nicotine addiction and cessation methods.
- The FDA gained access to product research previously hidden by companies.
- Independent scientific review panels influenced regulatory decisions.
- Research supported stronger warnings and policy adjustments.
- Public availability of ingredient data advanced health research.
Positive vs. Negative Impacts
| Aspect | Positive Impacts | Negative Impacts |
|---|---|---|
| Public Health | Lower youth smoking, higher awareness, fewer misleading claims | Slow reduction in adult smoking rates |
| Industry | Safer product oversight | Loss of profits, higher compliance costs |
| Society | Reduced normalization of smoking, long-term healthcare savings | Job losses in tobacco manufacturing |
| Regulation | Stronger federal oversight, transparency | Increased legal disputes with industry |
| Science | Better access to data, stronger research funding | Slow adaptation of policies to emerging products |
Long-Term Societal Impacts
- Tobacco’s cultural image shifted from glamour to health hazard.
- Communities witnessed greater involvement in smoking prevention.
- Generational smoking declines became visible in national surveys.
- Public spaces grew healthier due to stronger anti-smoking norms.
- Long-term reduction in disease burden created a healthier population outlook.
Key Takeaways
The Family Smoking Prevention and Tobacco Control Act of 2009 represented a landmark moment in the fight against tobacco-related harm. The law reshaped industry practices, empowered the FDA, and enhanced consumer protections. Public health improvements, particularly in reducing youth smoking, demonstrated the effectiveness of strong regulatory oversight. While challenges remained, such as industry resistance and the slow decline of adult smoking, the act set a precedent for health-centered policy. Future generations continue to benefit from the protections established through this transformative legislation.